A reagent
kit for detection and quantification of mRNAs of the
chimeric BCR-ABL (p190) mbcr transcript and reference ABL gene in
clinical material by polymerase chain reaction (PCR) with real-time
hybridization-fluorescence detection.
The kit can be
used to detect cases of Philadelphia chromosome-associated ALL, to confirm the
diagnosis of ALL, and to monitor the effectiveness of therapy, i.e., to
assess the
levels of minimal residual disease (MRD).
The kit of
reagents is designed for studying 24 clinical samples in quantitative format in
duplicate (a
total of 132 PCR reactions, including controls).
Additional
information about the molecular marker:
The
chimeric gene BCR-ABL is formed as a result of the rearrangement of the long
arms of chromosomes 9 and 22, which also leads to formation of the so-called
Philadelphia chromosome (t(9;22), Ph). This translocation is characteristic of
chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL), and it may also occur in acute myeloid
leukemia (AML). The chimeric BCR-ABL gene encodes a protein with tyrosine
kinase activity and plays an important role in the development of leukemia. There are several variants of
the BCR-ABL gene transcripts that are characteristic of different types of
leukemia. Thus, CML is characterized by transcripts b3a2 and b2a2, leading to
the formation of a protein with a molecular weight of 210 kDa (p210 transcript,
Mbcr), while most children with Ph+ ALL have a chimeric e1a2 transcript
encoding a protein with a molecular weight of 190 kDa (p190 transcript, mbcr).
Both BCR-ABL mbcr and BCR-ABL Mbcr transcripts are also found in a number of
adult ALL patients.
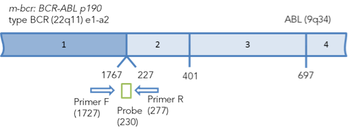
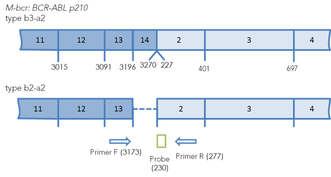
Figure 1.Arrangement of chimeric BCR-ABL transcripts b3a2, b2a3 and e1a2. The binding sites of primers and probes for real-time PCR are marked. The positions of primers and probes are indicated relative to the 5' end of the nucleotide sequences of normal transcripts.
The use of real-time PCR (Real-Time Quantitative PCR, RQ-PCR) to assess the level of BCR-ABL chimeric transcript expression in the studied cells makes it possible to achieve a significantly higher sensitivity in assessing minimal residual disease (MRD), detecting one leukemic cell per 50,000 normal leukocytes.
More detailed
information on diagnostic approaches, frequency of the study and prognostic value of this testing in
leukemias are available on the website of the International Network European
Leukemia Net (http://www.leukemia-net.org).
Related publications:
- GabertJ, Beillard E et al. Standardization and quality control studies of real-time quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia – a Europe Against Cancer program. Leukemia. 2003 Dec;17(12):2318-57.
- Baccarani, M. et al. (2009) Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J. Clin. Oncol. 27, 6041.
- Branford, S. et al. (2006) Rationale for the recommendations for harmonizing current methodology for detecting BCR-ABL transcripts in patients with chronic myeloid leukaemia. Leukemia 20, 1925.
- Hughes, T. et al. (2006) Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood 108, 28.
- van der Velden, V.H., Hochhaus, A., Cazzaniga, G., Szczepanski, T., Gabert, J., and van Dongen, J.J. (2003) Detection of minimal residual disease in hematologic malignancies by real-time quantitative PCR: principles, approaches, and laboratory aspects. Leukemia 17, 1013.
- Gabert, J. et al. (2003) Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia - a Europe Against Cancer program. Leukemia 17, 2318.
- Beillard, E. et al. (2003) Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using ‘real- time’ quantitative reverse-transcriptase polymerase chain reaction (RQ- PCR) - a Europe against cancer program. Leukemia 17, 2474.
Ordering information:
BCR-ABL1 mbcr RQ Kit (e1a2 transcript, p190), 24 tests Cat.No IG-RQ-2-24
Additiional reagents:
Blood RNA stabilizer medium Cat.No IG-RSB-100
TriZ Reagent Kit Cat.No IG-TRK-100
ReverZyme Kit Cat.No IG-RT-1
Erythrocyte Lysis Solution Cat.No IG-TRL-100
Price: on request
Reagents are for research use only (RUO)